Cyclo Olefin Polymer for Pharmaceutical Applications
Expertise and Performance Drive Innovation
Engineered for applications where purity, consistency, and performance are non-negotiable.

Medical Grade ZEONEX® and ZEONOR® Advantages
Compliant with USP class VI and <661.1>, ISO 10993, and various Pharmacopeia regulations (EP, JP), ZEONEX® and ZEONOR® Medical and Pharmacopeia Grade resins, made of Cyclo Olefin Polymer (COP), are the ideal choice for pharmaceutical drug storage and drug delivery devices.
- Superior drop resistance vs. glass
- Mechanically tough and durable, even at cryogenic temperatures
- Low protein adsorption
- Low moisture permeability
- High purity
- Compatible with a range of sterilization methods
- FDA Drug Master File (DMF) available upon request
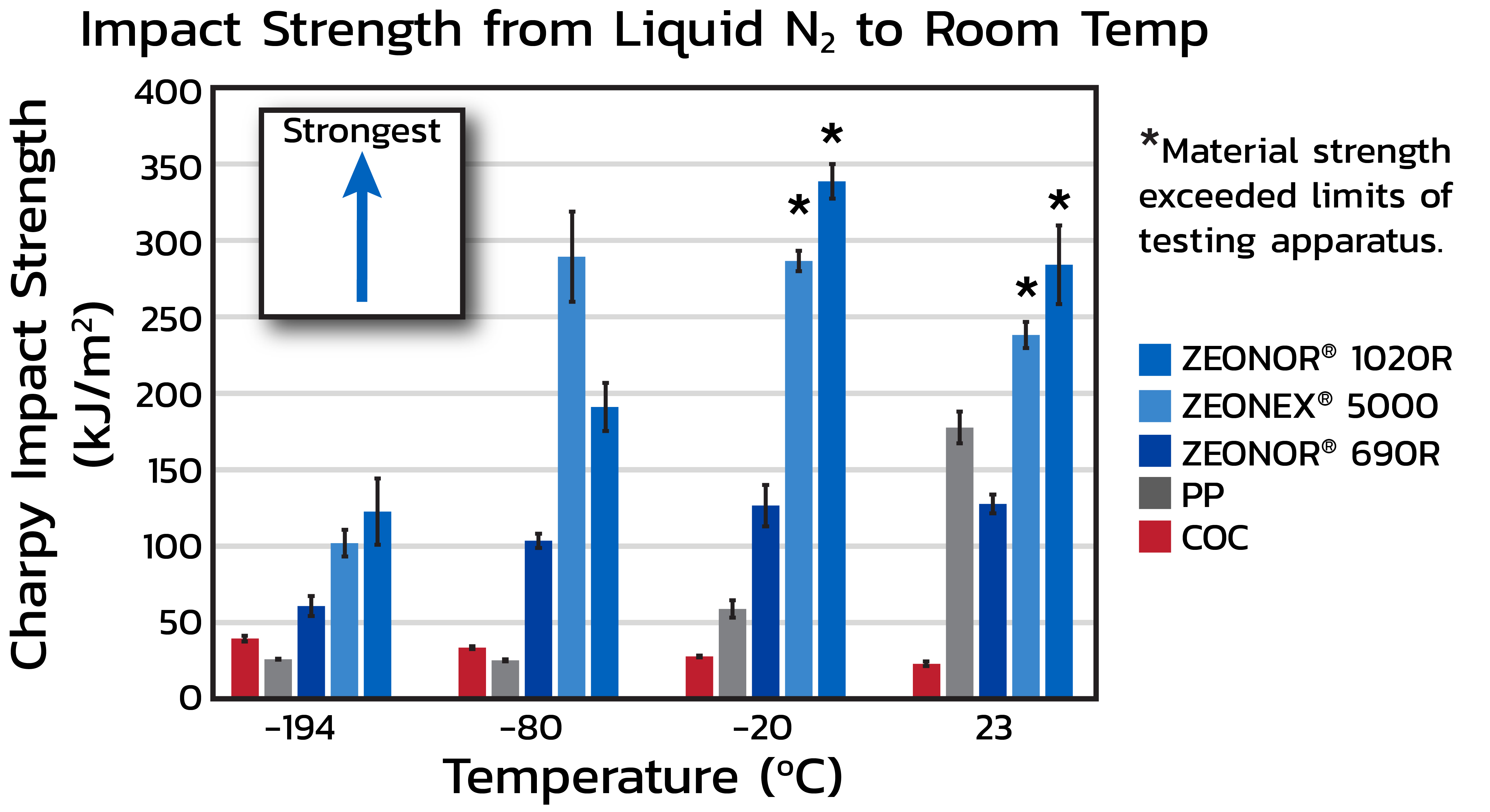
Durable from cryogenic to steam sterilization
Medical-grade ZEONEX® and ZEONOR® Cyclo Olefin Polymer (COP) have superior impact strength over a wide range of temperatures. Compared to polypropylene (PP) and COC, ZEONEX® and ZEONOR® COP is durable even at cryogenic conditions (-194 oC), providing dependable protection for pharmaceuticals during cold-chain storage. ZEONEX® and ZEONOR® COP is also mechanically tough and can withstand increased stress from viscous drug formulations and auto-injector devices.
Purity
Low Moisture Permeation
Low protein adsorption
Sterilization compatible
*Only 690R and 790R are compatible with steam sterilization
Technical Highlights
| Unit | Test | ZEONEX® 690R | ZEONEX® 790R | ZEONOR® 1020R | ZEONEX® 5000 | |
|---|---|---|---|---|---|---|
| Pharmacopeia registerred | Yes | Yes | Yes | Yes | ||
| Properties | ||||||
| Melt flow rate (MFR) (280 ⁰C, 21.18 N) | g/10min | JIS K719, ISO 1133 | 16.9 | 6.7 | 19.6 | 8.4 (230 ⁰C) |
| Glass transition temperature (Tg) (20 ⁰C/min) | ⁰C | JIS K7121 | 136 | 161.7 | 101.7 | 68.9 |
| Deg. of light transmission (t = 3mm) | % | ASTM D1003 | 91.5 | 91.5 | 91.5 | 91.4 |
| Sterilization Compatibility | ||||||
| Ethylene Oxide | Yes | Yes | Yes | Yes | ||
| Gamma irradiation | Yes | Yes | Yes | Yes | ||
| Steam / autoclave | Yes | Yes | No | No | ||
| Vaporized hydrogen peroxide | Yes | Yes | Yes | Yes | ||
| Pharmaceutical Storage Compatibility | ||||||
| Surface reactivity | Inert | Inert | Inert | Inert |
Applications
Engineered to Perform
Pre-Filled Syringes

Vials

Wearable Drug Delivery Devices
Certifications




Contact Us
Partner with Experts
Our team goes beyond the sale to provide informed, technical solutions. Let us guide your innovation.
Start Your Project